By Duncan B. Will, CPA/ABV/CFF, CFE
The AICPA’s Statements on Quality Management Standards’ (“SQMS”) December 15, 2025, effective date is fast approaching, and few firms are prepared. Since the SQMS were issued in June 2022, the AICPA has produced a plethora of related content. This article is not intended as a treatise on the SQMS. Instead, this article is an extremely brief overview of the SQMS, and shares risk management tips intended to motivate you to make progress on modifying your firm’s system of quality management to be compliant with the SQMS.
Overview
The SQMS represent a significant overhaul of the quality control framework for CPA firms, shifting from a rules-based to a risk-based quality management framework. These standards are designed to ensure that firms establish, implement, and maintain effective systems of quality management tailored to their unique circumstances and engagements.
The items listed and in the image, below, are components of the new SQMS, initially introduced when the International Auditing and Assurance Standards Board rolled out their quality management standards. Three components are elements of the extant quality control standards (“QCS”); three are renamed and expanded/updated elements of the (soon to be replaced) QCS; and the new components are the two COSO components not addressed in the extant QCS.
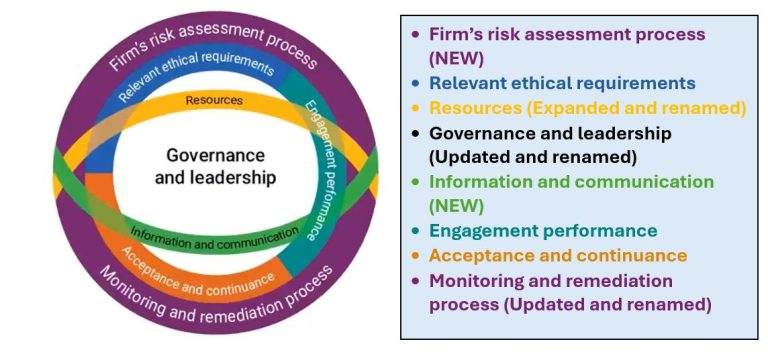
You are not required to implement the standards before December 15, 2025, but all standards applicable to your firm must be implemented by that date. Firms performing services in accordance with Statements on Auditing Standards, Statements on Standards for Accounting and Review Services, or Statements on Standards for Attestation Engagements must design and implement their systems of quality management in compliance with the SQMS and should already have begun this process.
The initial evaluation of your firm’s designed system of quality management is to be performed during the year ending December 15, 2026, and annually thereafter, to assess whether your system of quality management meets its quality objectives.1
Risk Management Tips:
The clock is ticking, and December 15, 2025, is fast approaching, so although not meant to be all-inclusive, do consider the following risk management tips and best practices:
- Don’t let “perfect be the enemy of good.” If unchecked, this aphorism can create crippling inertia in the development of your quality management process. Understand that a system of quality management is an evolving, iterative, dynamic process. Learn from the process, share information with your team, and use that information to improve your system of quality management.
- Seek your peer reviewer’s guidance with the transition. Your peer reviewer’s familiarity with your quality control system and understanding of the new standards can be instrumental in assisting you with designing your system of quality management. Ask what tools your peer reviewer believes would be beneficial for you. Ideally, you can obtain your peer reviewer’s insight and tips specific to your unique needs. However, be cautious not to rely too heavily on your peer reviewer (unless you are willing to secure the services of another) as doing so could threaten your peer reviewer’s independence.
- Consider having a senior member of your system of quality management development team lead your firm’s brainstorming sessions and adopt a two-phased approach to brainstorming. During the initial phase, the discussion leader should encourage and reinforce that this phase is exclusively for the generation of ideas, and that there be no evaluation or criticism of ideas raised. Care should be taken to record every suggestion. Only during the second phase should the team evaluate or constructively critique aspects of the initial brainstorming phase. This two-phase approach will encourage team members to offer a greater number of suggestions as well as more-nuanced suggestions, which might otherwise not be captured and considered in the development of your system of quality management.
- Consider supplementing your external resources by collaborating with other practitioners with similar practices.
- Don’t overcomplicate your transition process or try to address every potential risk. Instead, focus on the quality risks that are material, relevant, or of higher risk to your firm; the types of industries, businesses, and organizations you serve; and the services you offer.
- SQMS No. 1 indicates that root cause analyses should be performed by people responsible for the firm’s system of quality management. But to maintain objectivity, you should take care to avoid assigning individuals to perform root cause analyses that are on engagements being reviewed.
Root cause analysis can be extremely complicated, but don’t get lost. Keep it as simple as possible. Now may be a good time to reacquaint yourself with or to discover the “Five Whys Technique,” which involves repeatedly asking “why” to identify a problem’s root cause. The technique can help you and your firm evaluate and avoid overlooking the root causes of identified deficiencies in your firm’s system of quality management.
- As with the extant quality control standards, the SQMS require you to document your system of quality management. As before, that documentation may be used by your peer reviewer to assess whether your firm has complied with the standards. If documentation indicates your firm will perform procedures exceeding those required by professional standards, those elevated requirements will be the benchmark used to assess your compliance. So, take care in documenting your firm’s quality objectives, quality risks, your responses to those risks, and ultimately your system of quality management, taking care to identify those responsible and accountable for your system.
- Keep up to date with quality management standards’ developments and take advantage of resources developed and shared by the AICPA: A journey to quality management | Resources | AICPA & CIMA
- Firms encountering challenges with the transition should consider seeking external support. Firms may benefit from engaging entities that specialize in offering tailored assistance with the implementation of the quality management standards.
Contact CAMICO
CAMICO policyholders with questions regarding this communication or other risk management questions should contact the Loss Prevention department at lp@camico.com, or call our advice hotline at 800.652.1772 and ask to speak with a Loss Prevention Specialist.
1 Quality objectives — The desired outcomes in relation to the components of the system of quality management to be achieved by the firm.

